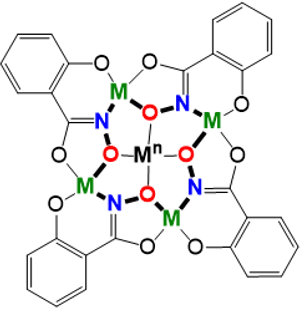
The bulk of the research in the Lutter Lab involves a class of coordination complexes known as metallacrowns. Metallacrowns are an inorganic analog to crown ethers that were first introduced by Pecoraro and Lah in 1989. Essentially, the [–C–C–O–]n repeating unit of a crown ether is replaced by a [–M–N–O–]n repeating unit, where M is usually a 3d transition metal and the N–O typically are provided by hydroximates. Like crown ethers, the central cavity of a metallacrown can bind to a wide variety of metal ions including—alkali metals, transition metals, and even lanthanides. Metallacrowns are synthesized via self assembly reactions and are amenable to variation of ring metal and/or hydroximate ligand choices. As a result, many variations of metallacrowns have been reported across a wide range of compositions and sizes. Rational design of metallacrowns have contributed to several fields of study including host–guest binding, molecular magnetism, trivalent lanthanide sensitization, and even magnetic resonance imaging. In addition, given that metallacrown synthesis is easy to learn, projects involving these compounds are accessible to undergraduate researchers of various backgrounds and experience.
Luminescence is the emission of a photon that does not result from heat. Electronic transitions in chemical compounds are the primary mechanism by which light and matter may interact to give luminescence. Our work has involved the luminescent properties of trivalent lanthanide ions that have valence electrons which are protected from their bonding environment. As a result trivalent lanthanide ions have characteristic and narrow emission bands with long lifetimes. These properties are attractive for optical imaging, however, these ions absorb light poorly. To counteract this issue, organic antenna are adorned to lanthanide ions, which in our case are the hydroximates in metallacrowns.
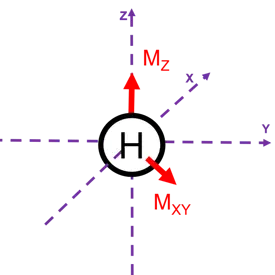
Magnetic resonance describes the phenomenon that arises from the Zeeman splitting of magnetic moments in an applied field. In magnetic resonance imaging, the magnetic moments arise from protons in water of biological systems. This nuclear magnetic resonance can be perturbed by radio waves to enter an excited state, which relax via longitudinal and transverse mechanisms. The time for these mechanism to return the resonance to its ground state can be altered by paramagnetic species such as gadolinium(III), europium(II), and iron(III) resulting in contrast.
Molecular magnetism is the examination of magnetic properties on the molecular scale. Our work in molecular magnetism has involved the synthesis and characterization of single molecule magnets (SMMs), and magnetocoolants. SMMs are superparamagnetic molecules that have a barrier between the spin up and spin down magnetic states. This barrier depends on the overall spin and magentoanisotropy of the molecule. In addition, a good SMM will have an isolated ground state. Conversely, a magnetocoolant performs well when the magnetic ground state is close in energy to multiple excited states. This configuration generates a more entropic ground state. The presence or absence of the entropy as an external field is applied or removed powers a cycle that can result in a thermal pump at cryogenic temperatures.
One of the older idioms in chemistry is that structure and function are related. Polarity, reactivity, and even spectroscopy of a given molecule all have a relationship with the structure. Single-crystal X-ray diffraction is a technique useful for structural determination of compounds. Given the structural complexity of metallacrown complexes, crystallographic data has been an essential tool for characterization of ligand fields of metal ions and intermolecular interactions in the solid-state of our complexes to help support observed phenomenon.
2024–2025: Early Career Faculty Research Grant, University of Southern Indiana, $5000
2023–2024: Science, Engineering, and Education Research Grant, Pott College, $5000
2025: Cambridge Isotope Labs, Inc. Research Grant, $2888
2026–2027: Science, Engineering, and Education Research Grant, Pott College, $5000